Valeda Demonstrates Improvements in Clinical and Anatomical Outcomes Supporting a Disease-Modifying Benefit
LIGHTSITE III
Double-masked, randomized, sham-controlled, parallel group, multi-center trial to assess the safety and efficacy of photobiomodulation (PBM) treatment with Valeda in subjects with dry age-related macular degeneration (AMD)
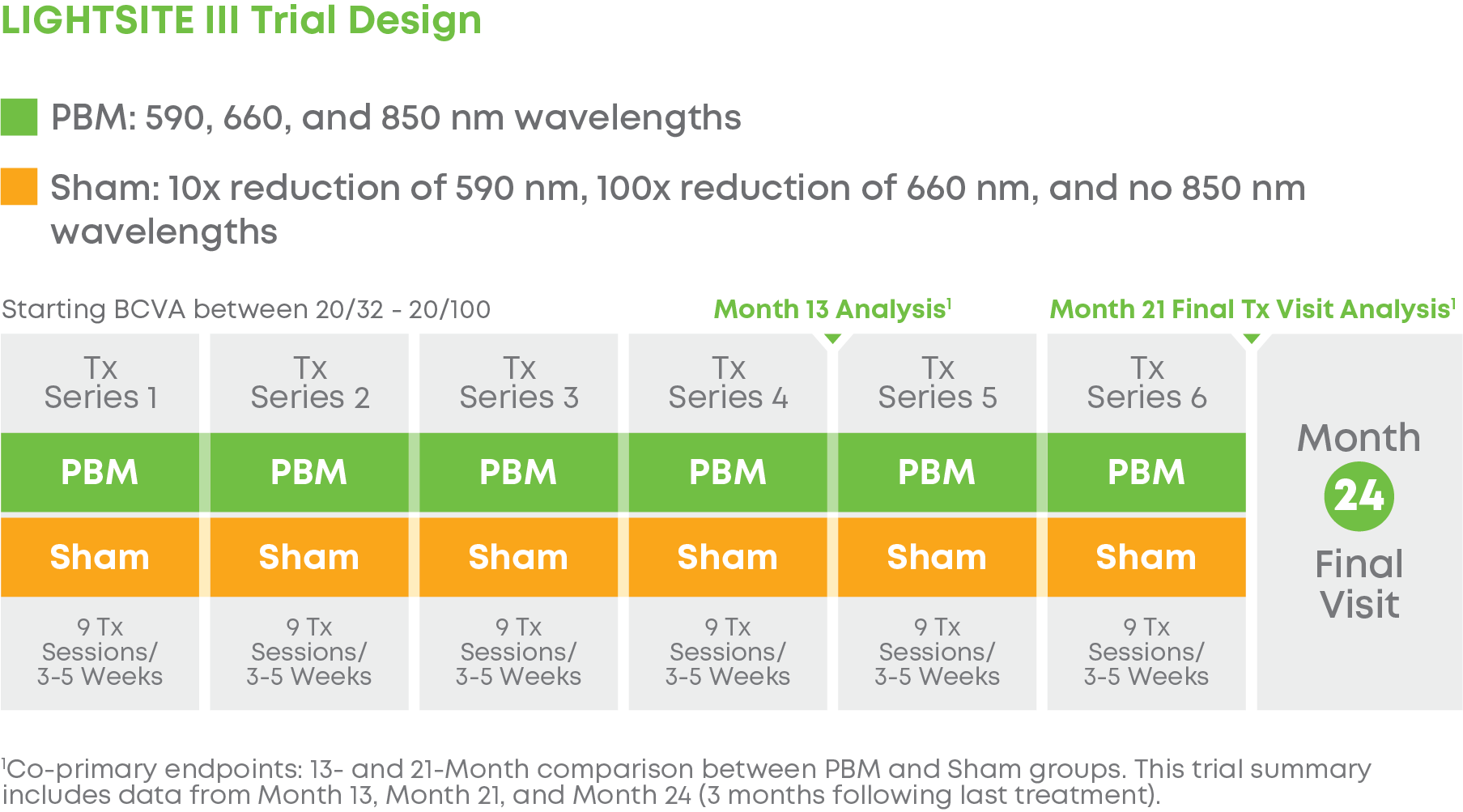
The LIGHTSITE III study evaluated the safety and efficacy of photobiomodulation (PBM) for the treatment of dry age-related macular degeneration (AMD). The study aimed to evaluate the long-term vision and anatomical benefits of PBM using the Valeda Light Delivery System in subjects with dry age-related macular degeneration (AMD).
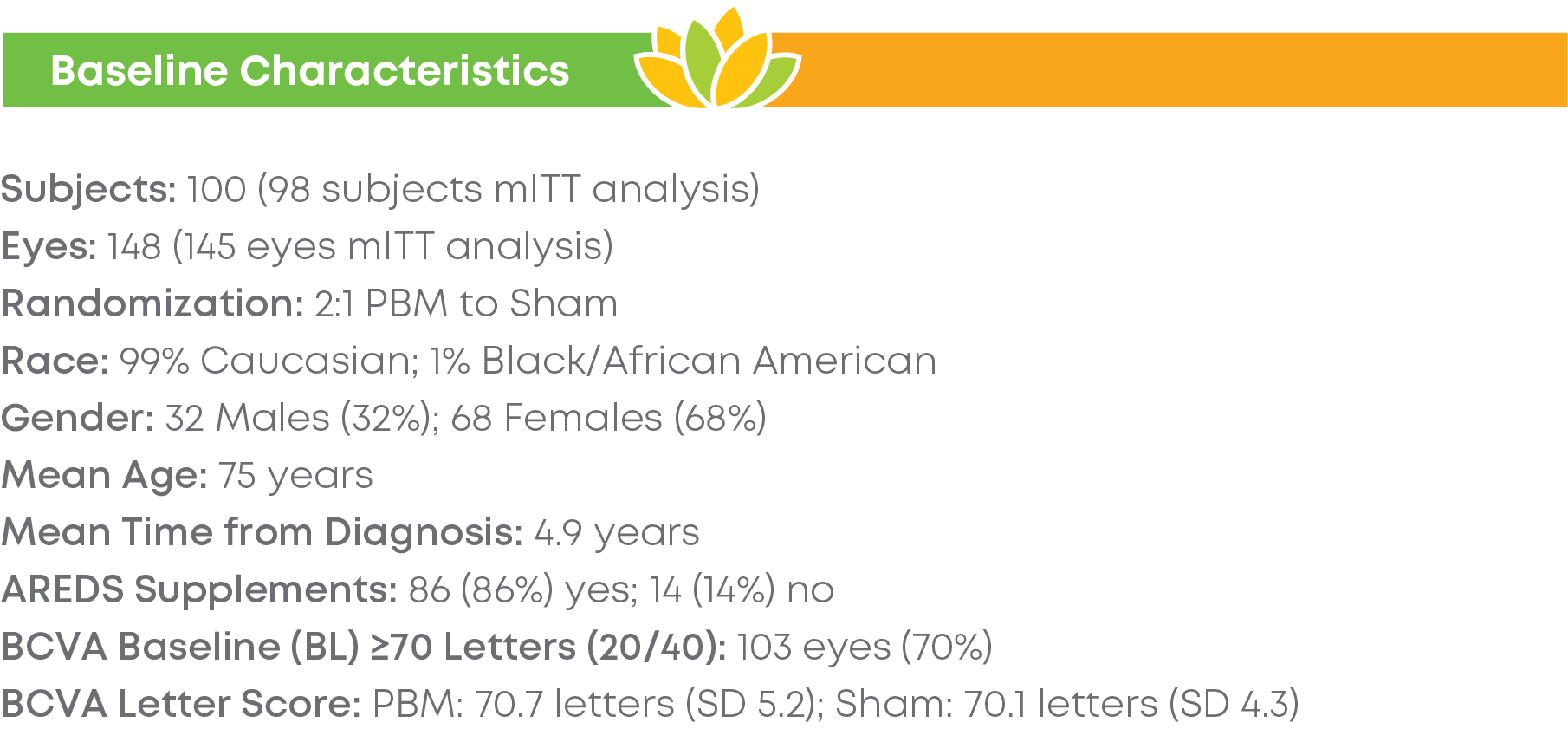
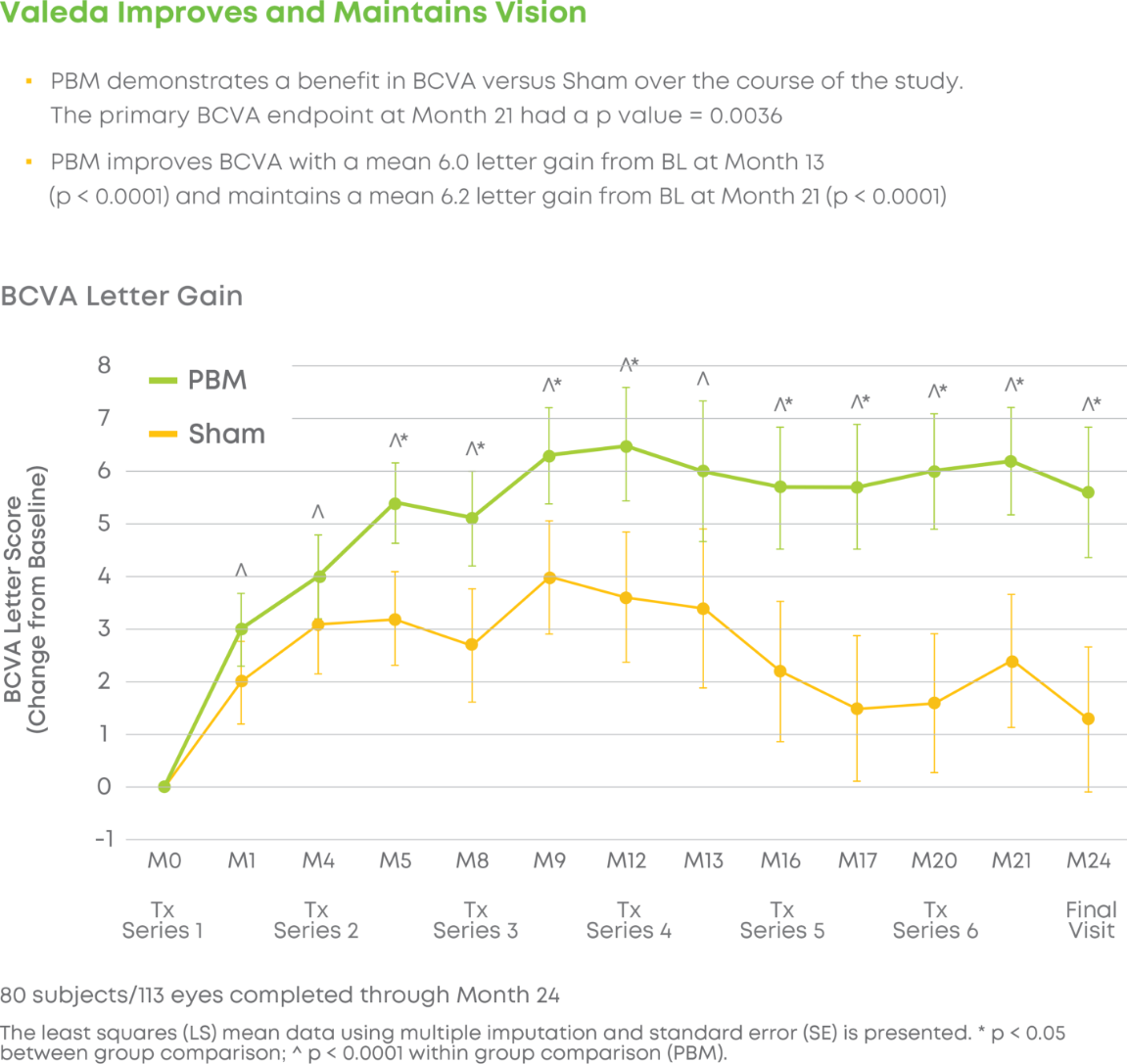
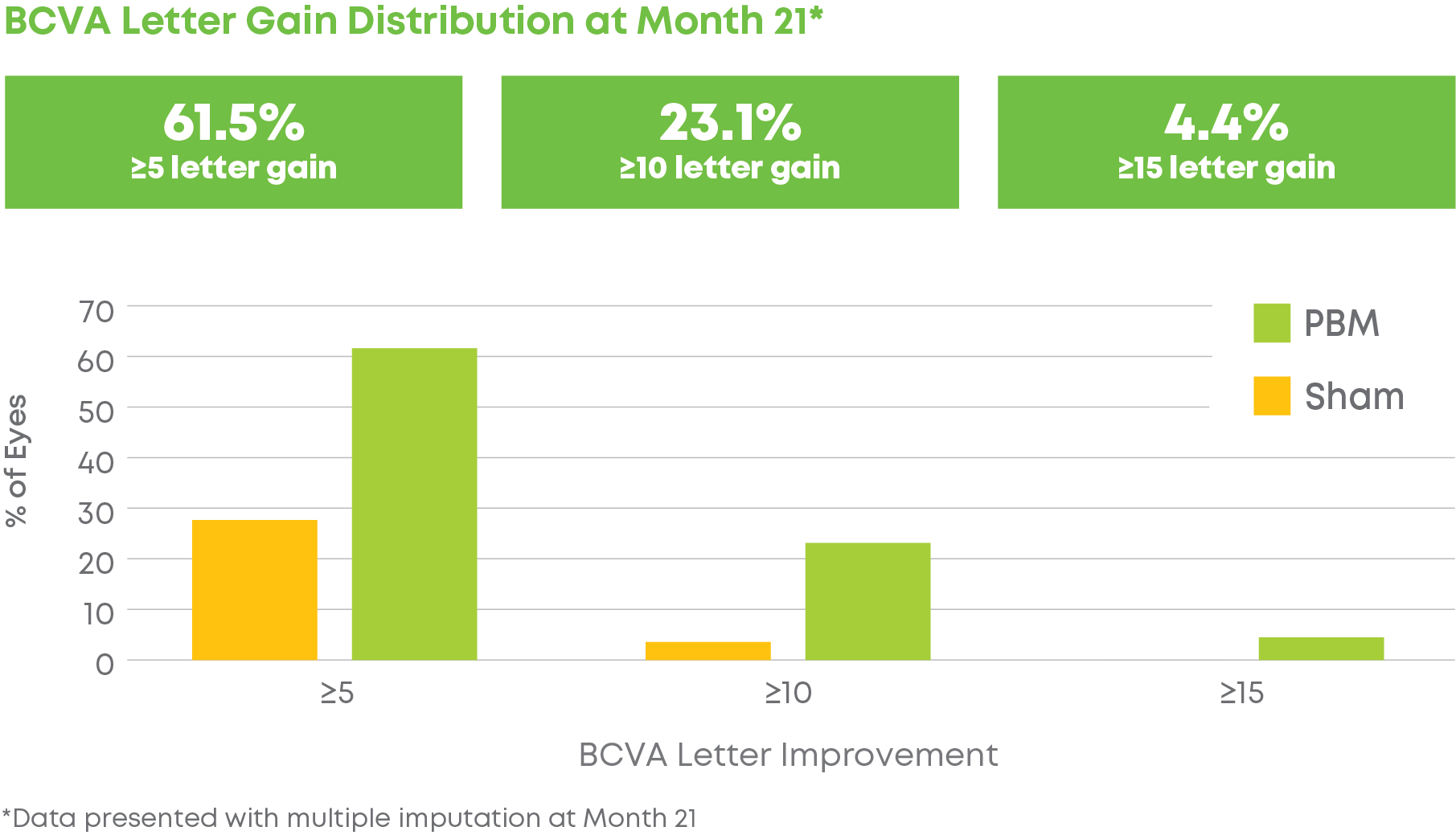
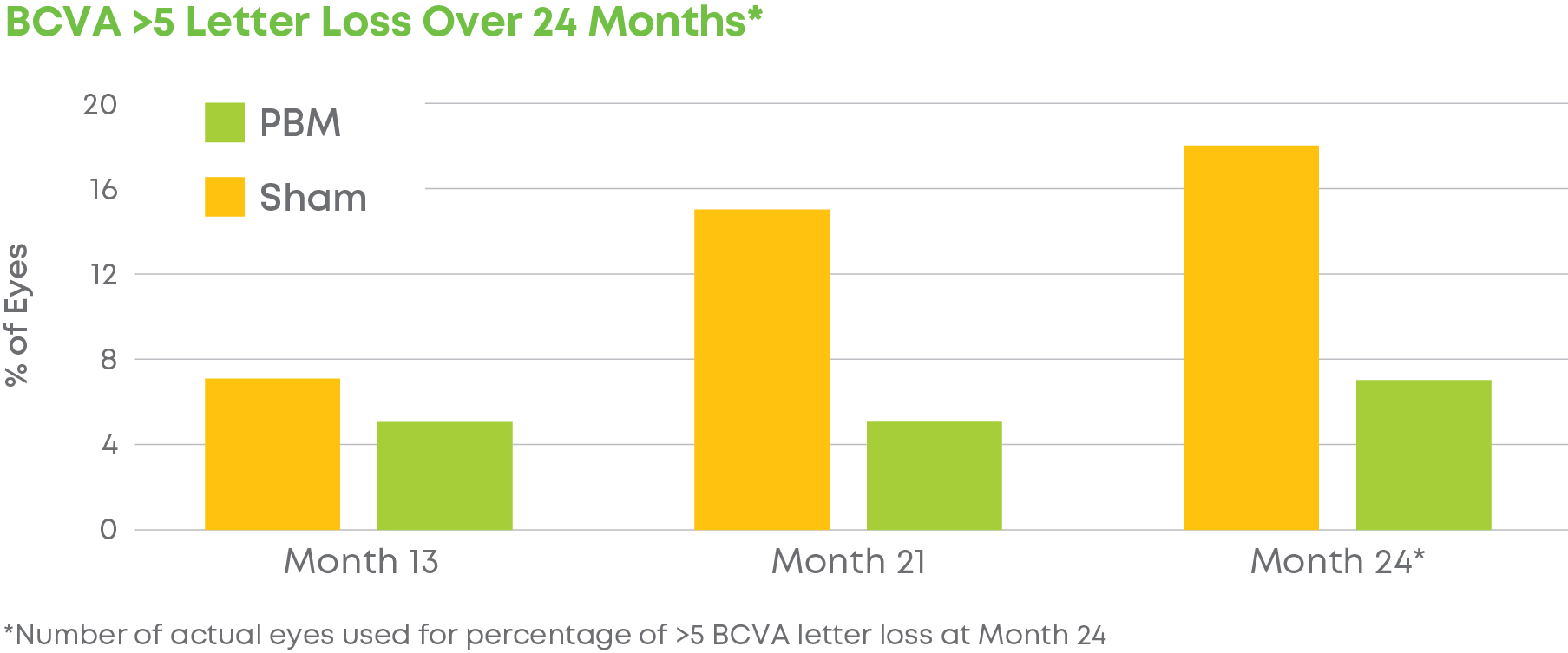
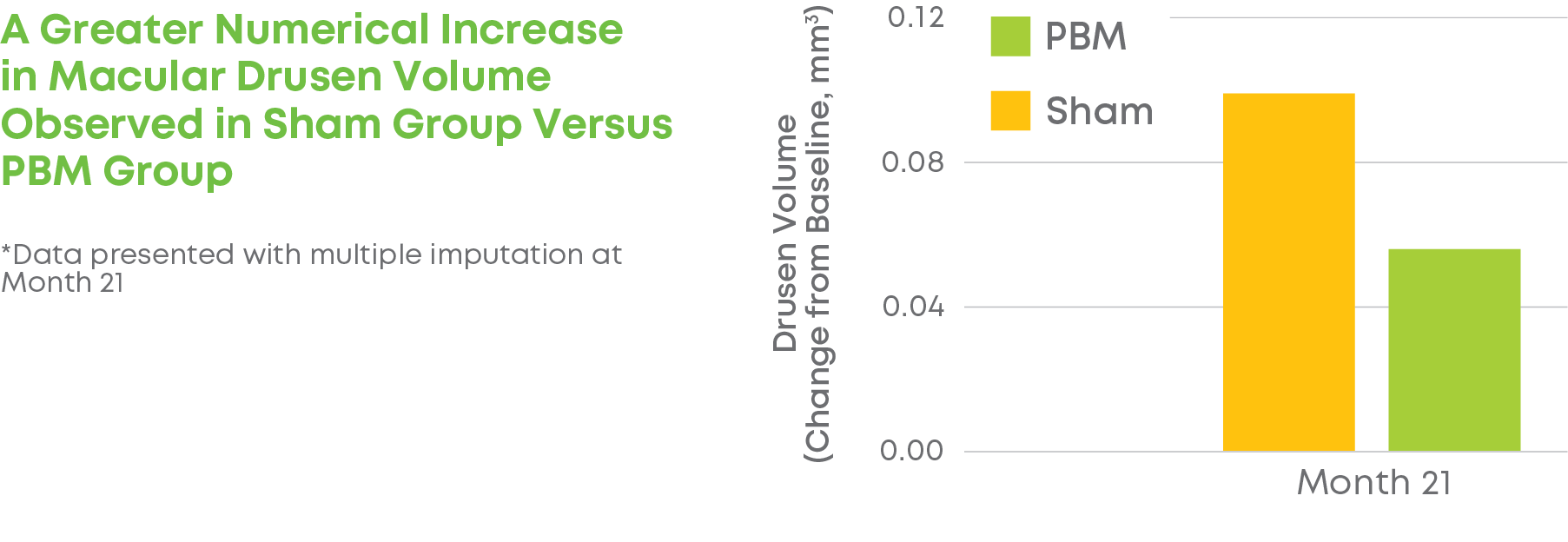
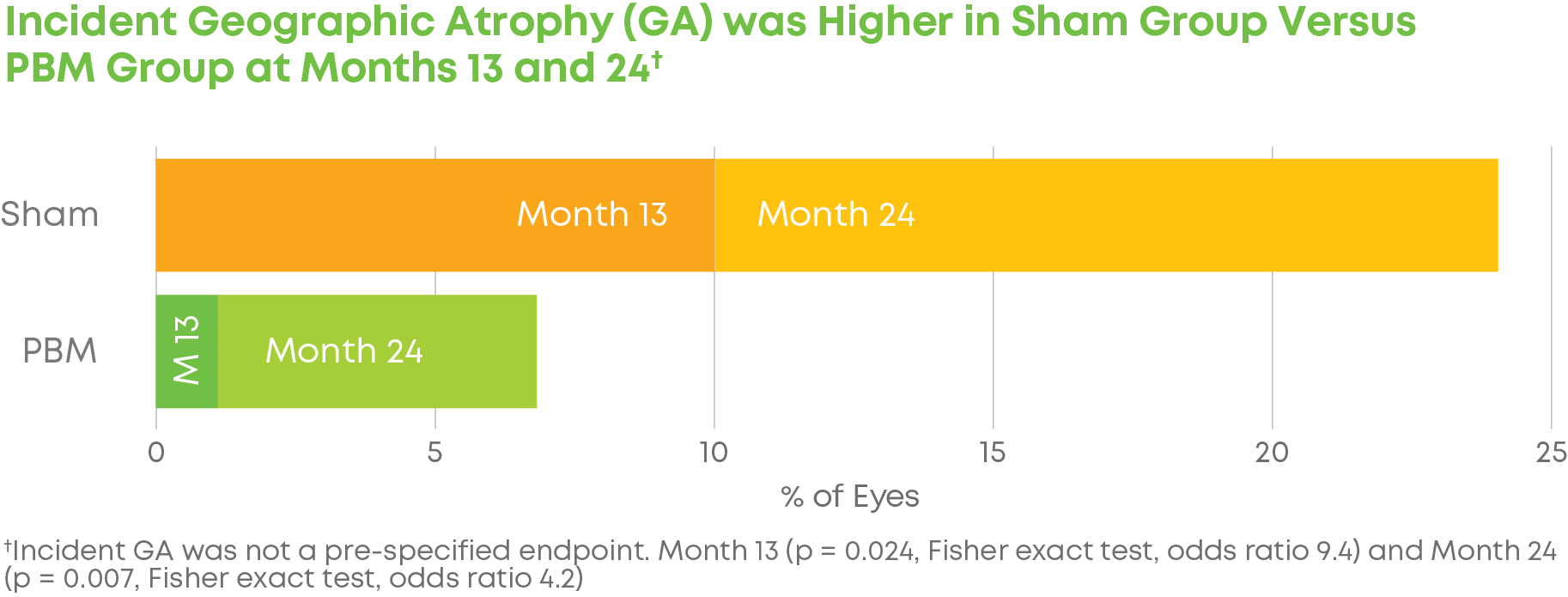
Subjects were randomized into a 2:1 treatment groups. Each group received 3 treatments per week over 3-5 weeks every four months for a total of 24 months. The trial included 148 eyes (100 subjects) and participation lasted 24 months. LIGHTSITE III met the predetermined primary efficacy of BCVA with a statistically significant difference between PBM and Sham groups with a gain of 6.2 letters at Month 21 (p = 0.0036), which was maintained at Month 24 with a gain of 5.6 letters. At Month 21, approximately 61.5% of PBM-treated eyes showed a ≥ 5 letter gain (mean of 9.0 letters), 23.1% of PBM-treated eyes showed a ≥ 10 letter gain (mean of 12.8 letters), and 4.4% of PBM-treated eyes showed a ≥ 15 letter gain (mean of 15.5 letters). Incident geographic atrophy (GA) was reduced in the PBM compared to the Sham group at 24-months (p = 0.007; PBM, 6.8% vs. Sham, 24.0%). Although incident GA was not a pre-specified clinical endpoint, the results support overall safety benefits of treating earlier in dry AMD disease. A favorable safety profile was observed.
Valeda Light Delivery System User Manual. LumiThera, Inc.
A favorable safety profile was observed with no signs of phototoxicity
LumiThera Sponsored Trials
The LIGHTSITE I (NCT02725762) study is the first prospective, sham-controlled, double-masked clinical study to evaluate the effectiveness of photobiomodulation (PBM) treatment in patients with dry age-related macular degeneration (AMD) using the Valeda® Light Delivery System [Canada]. Subjects were to be treated with 2 series of multiwavelength PBM or Sham treatment delivered 3x per week over 3-4 weeks every 6 months. The study included 46 eyes (30 subjects) and subject participation lasted 12 months. Results from the LIGHTSITE I study showed clinical improvements in visual and anatomical outcome measures following multiwavelength PBM. Improvements were observed in Best-Corrected Visual Acuity (BCVA), Contrast Sensitivity (CS), microperimetry, and Quality of Life (QoL) in addition to improvements in pathological hallmarks of disease including central drusen volume and thickness. No device-related adverse events were reported throughout the course of the study highlighting a favorable safety profile. These data provided a strong foundation for the utility of PBM therapy and demonstration of disease-modifying effects prompting additional studies.
The PBM therapy was most beneficial in dry AMD subjects immediately following the completion of each treatment series and lost some benefit throughout the 6-month treatment interval, highlighting a need for follow-up maintenance therapy. The LIGHTSITE I results were used to refine the subsequent multi-center clinical trials in Europe (LIGHTSITE II) and the USA (LIGHTSITE III).
A Double-Masked, Randomized, Sham-Controlled, Single-Center Study with Photobiomodulation for the Treatment of Dry Age-Related Macular Degeneration [Markowitz SN, Devenyi RG, Munk MR, et al. A DOUBLE-MASKED, RANDOMIZED, SHAM-CONTROLLED, SINGLE-CENTER STUDY WITH PHOTOBIOMODULATION FOR THE TREATMENT OF DRY AGE-RELATED MACULAR DEGENERATION. Retina. 2020;40(8):1471-1482. doi:10.1097/IAE.0000000000002632]
The LIGHTSITE II (NCT03878420) study is a double-masked, sham-controlled, parallel design, prospective, multi-site study for the use of PBM as a treatment for visual impairment in subjects with dry AMD [Europe]. Subjects were to be treated with 3 series of multiwavelength PBM or Sham treatment delivered 3x per week over 3-5 weeks every 4 months. The study aimed to enroll up to 144 eyes (96 subjects) in up to 16 sites across Europe. The study included 53 eyes (44 subjects) across 7 sites and subject participation lasted 10 months. The LIGHTSITE II study was underway when the COVID-19 viral outbreak occurred. This global public health emergency significantly impacted the conduct of the clinical trial. LumiThera put the study on hold for 11+ weeks and then decided to terminate new subject enrollment permanently and converted the study to a feasibility study. While COVID-19 had a significant interference on study design, improvement in visual outcomes in patients that completed all rounds of PBM treatment were observed. PBM-treated eyes showed statistically significant improvement in BCVA at Month 9 (p = 0.02) with a ~4-letter gain versus a 0.5-letter gain in the Sham group (p = 0.10). Approximately 35.3% of PBM-treated eyes showed ≥ 5-letter improvement at 9 months. While PBM and Sham groups both showed GA lesion growth in the trial period, there was 20% less growth in the PBM group over 10 months, suggesting potential disease-modifying effects. No safety concerns or signs of phototoxicity were observed. Overall, these findings further support the data from the LIGHTSITE I study.
LIGHTSITE II Randomized Multicenter Trial: Evaluation of Multiwavelength Photobiomodulation in Non-exudative Age-Related Macular Degeneration [Burton B, Parodi MB, Jürgens I, et al. LIGHTSITE II Randomized Multicenter Trial: Evaluation of Multiwavelength Photobiomodulation in Non-exudative Age-Related Macular Degeneration. Ophthalmol Ther. 2023;12(2):953-968. doi:10.1007/s40123-022-00640-6]
The ELECTROLIGHT (NCT04522999) is an open label, prospective, pilot clinical study to assess the effects of PBM and electroretinography (ERG) in subjects with Dry AMD. ERG is a quantitative and functional measure of visual output in the diagnosis and management of AMD. Subjects were treated with one series of multiwavelength PBM treatment delivered 3x per week over 3-4 weeks. The study included 23 eyes (15 subjects) at a single site and subject participation lasted 6 months. ERG protocols included multi-focal (mf-ERG), and multi-luminance (ml-ERG). Significant improvements of visual output were observed over the 6-month time course. Subjects showed an improvement in ml-ERG that was additive across the 9 PBM treatment sessions. PBM improved ml-ERG magnitude by 14.4% at Month 1 with a reduction to near baseline (BL) levels at Month 3. A moderate improvement in ml-ERG was observed at Month 6. PBM-treated subjects showed consistent improvements in BCVA following PBM treatment and a sustained vision improvement at Month 6 (p < 0.001). An approximate 12 letter (BCVA) vision improvement at Month 1, 3 and 6 was observed (p < 0.001). Significant improvement in contrast sensitivity and in the Amsler grid test were also observed. Valeda showed a positive safety profile with no signs of phototoxicity and a low rate of adverse events. These results support the use of PBM in dry AMD patients and the utility of ERG as a functional indicator for changes in retinal output.
Registration page: https://clinicaltrials.gov/study/NCT04522999?term=Electrolight&rank=1
Ongoing Clinical Trials
For information on studies and site locations visit www.clinicaltrials.gov.
